
F2 Lewis Structure, Molecular Geometry, Hybridization, Polarity, and MO
Molecular Orbital (MO) Diagram for F2 (2+) 28,328 views 9.5 Molecular Orbital Theory | General Chemistry Chad's Prep When two fluorine atoms bond, the sigma (2p) bonding molecular orbitals are.
Molecular Orbital Diagram For F2 Wiring Site Resource
For the ion F2+:a) Draw the molecular orbital diagram.b) Calculate the bond order.c) Would this ion exist?d) Write the electron configuration of the ion.————.

How to draw Molecular Orbital Diagram for F2 Molecular Orbital Theory
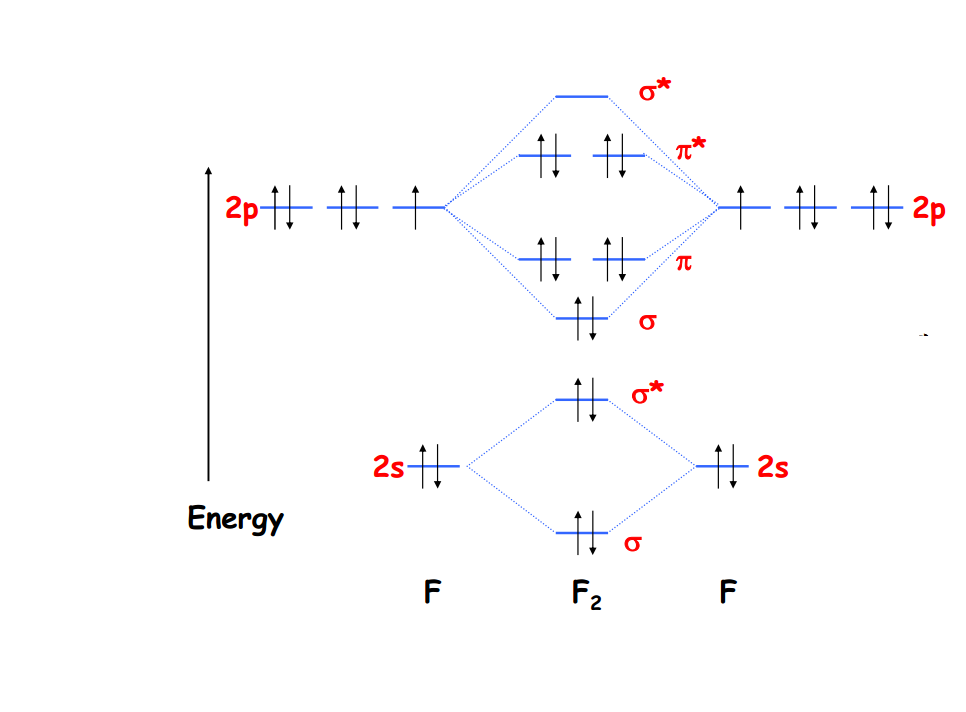
According to our diagram, there are 8 bonding electrons and 6 antibonding electrons, giving a bond order of (8 − 6) ÷ 2 = 1. Thus F 2 is predicted to have a stable F-F single bond, in agreement with experimental data. Figure 9.8.1: Molecular Orbital Energy-Level Diagrams for Homonuclear Diatomic Molecules. (a) For F 2, with 14 valence.

F2 Lewis Structure, Molecular Geometry, Hybridization, Polarity, and MO
Now let's put these ideas together to make an MO diagram for HF. We need to know what orbitals we are using. We are only going to consider valence orbitals. H has a 1s orbital. F has a 2s orbital and 3 2p orbitals (x,y,z). We want to know the energies of the orbitals.
[Solved] . Here is a partial MO diagram of F2. F F2 F. 0++0 2p (P..Py
This frequency was calculated to be 1270.63 cm-1. F2 Vibration The Highest Occupied Molecular Orbital (HOMO) is shown by clicking the button below. It was calculated using the aug-cc-pVTZ basis set. This correspondes to the 2p pi* orbital of the MO diagram. F2 HOMO

Draw the molecular orbital diagram for F2 and find out the bond order
Molecular Orbital Theory MO bonding in F2 and O2
Molecular Orbital Diagram For F2 Wiring Site Resource
2. I wanted to ask a question about notations on MO diagrams. Our first lesson about MO diagrams was today and I was taken through an example with FX2 F X 2 as shown below: but one thing that the lecturer did not explain, and I couldn't find over the internet with explanation, was the notation 3σXg 3 σ X g, 1πXu 1 π X u, 1πXgX∗ 1 π X g.

F2 MO Diagram YouTube
#3. Draw the MO diagram for `O_2^+` This is a bit of a curveball, but a perfectly valid problem. Recall that a cation indicates a loss of `1` electron. `O_2^+` is just the ionized form of `O_2`; that is, it's `O_2` with `1` missing electron. The MO diagram will be the same as the MO diagram of `O_2`, except with `1` less electron.

[Expert Answer] Draw the molecular orbital diagram for F2 and find out
Draw diagram of forming of sigma and pi bond of C 3 H 4 there is a. The bond lengths are inverse to the bond order, so the order is F2+ < F2 .There is 1 unpaired electron in F2+, 0 unpaired electrons in F2. Draw the \(\sigma\) and \(\sigma^*\) molecular orbital of \(CO\). Draw the MO energy level diagram and write the electron coefficient.
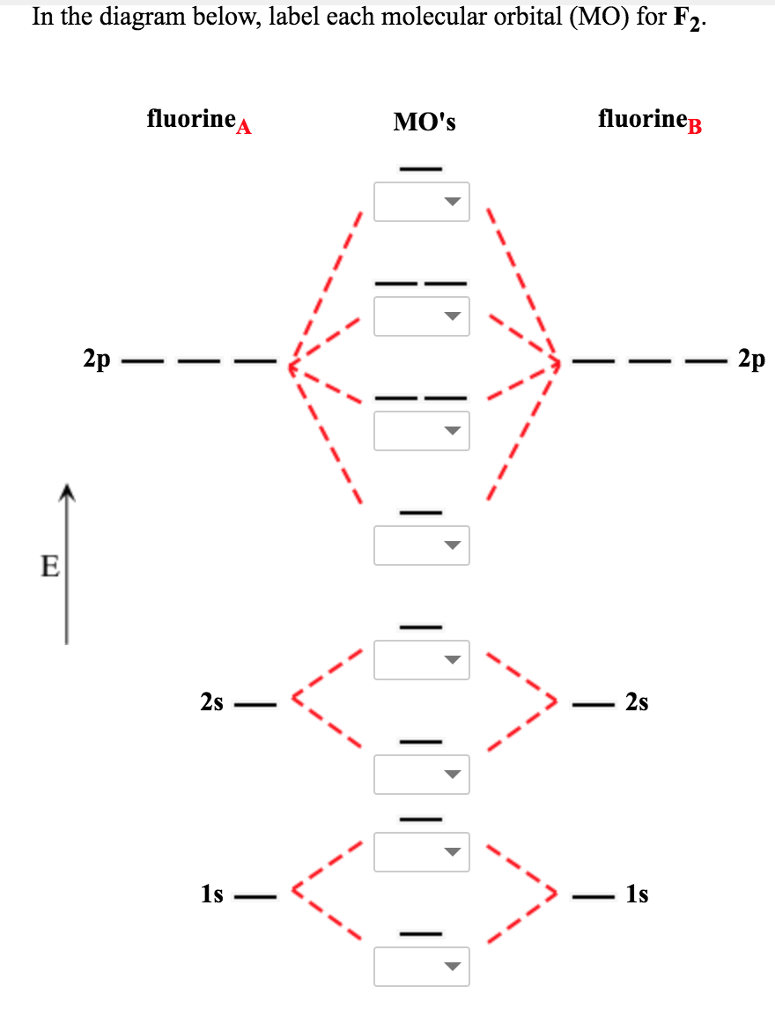
43 molecular orbital diagram f2
0:00 / 4:36 Molecular Orbital Diagram for F2 and F2+ Brandon C 7 subscribers Subscribed 5.6K views 3 years ago Here is a video that discusses over the Molecular Orbital Diagram for F2+.
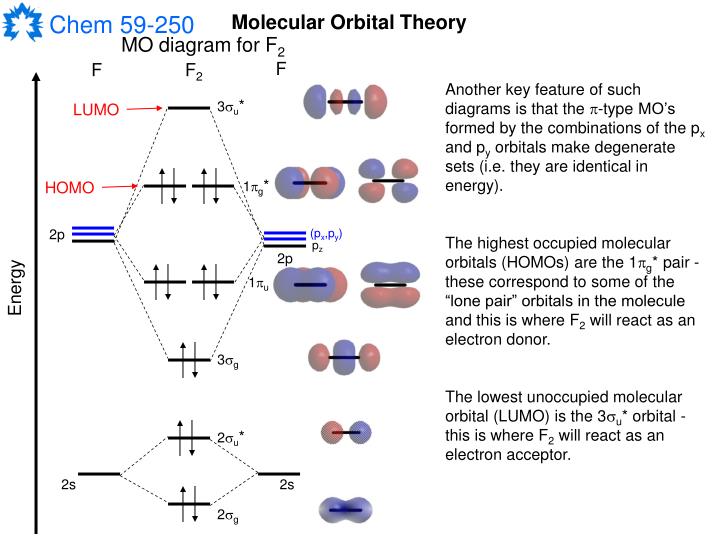
PPT The Delocalized Approach to Bonding Molecular Orbital Theory
A bare molecular orbital diagram is presented and you must drag the correct orbitals and labels onto the diagram. The diagram is then completed by filling the energy levels with the correct number of electrons. The following molecules are currently available: Molecules of the First Row: s and p-block Homonuclear Diatomic Molecules.
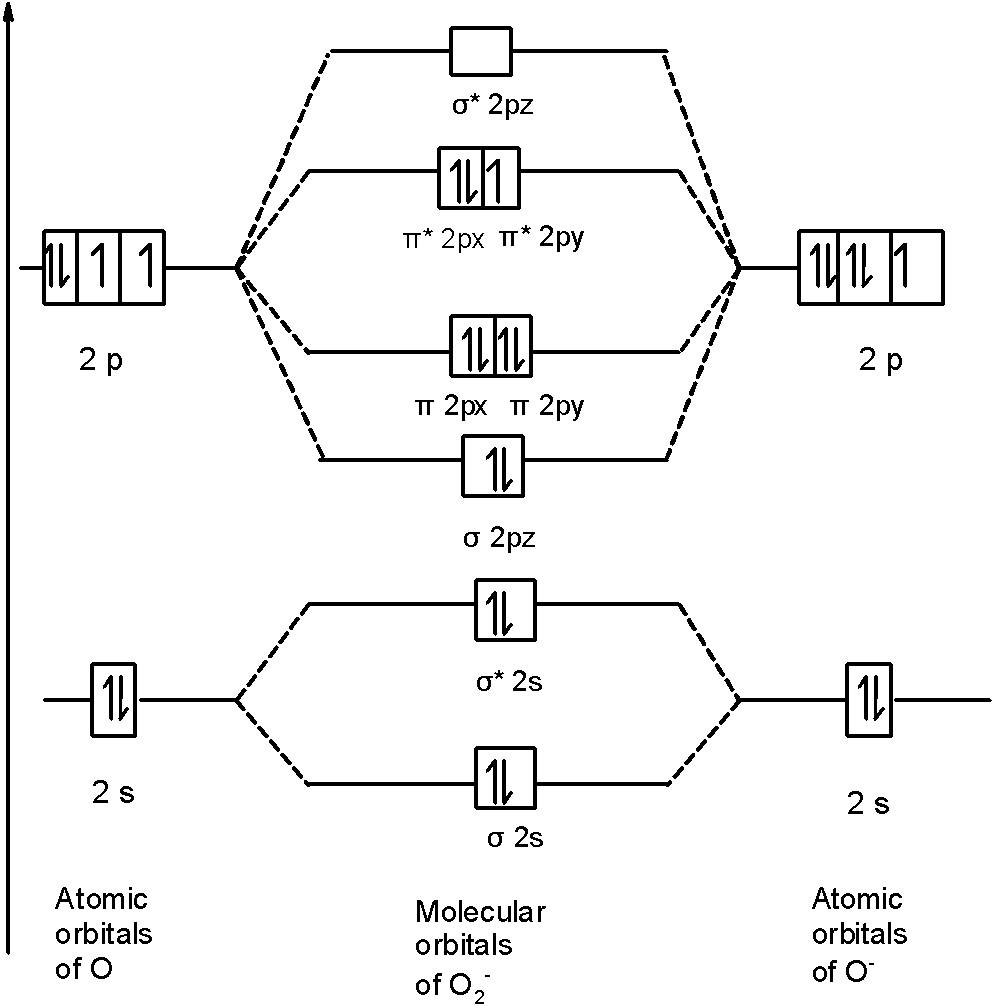
43 molecular orbital diagram f2
The interactive videos focused on qualitative quantum physical basics (F1) of the theory as well as practical applications (F2) i.e., the construction and interpretation of so-called MO diagrams to describe bond states between two or more atoms in a chemical compound.
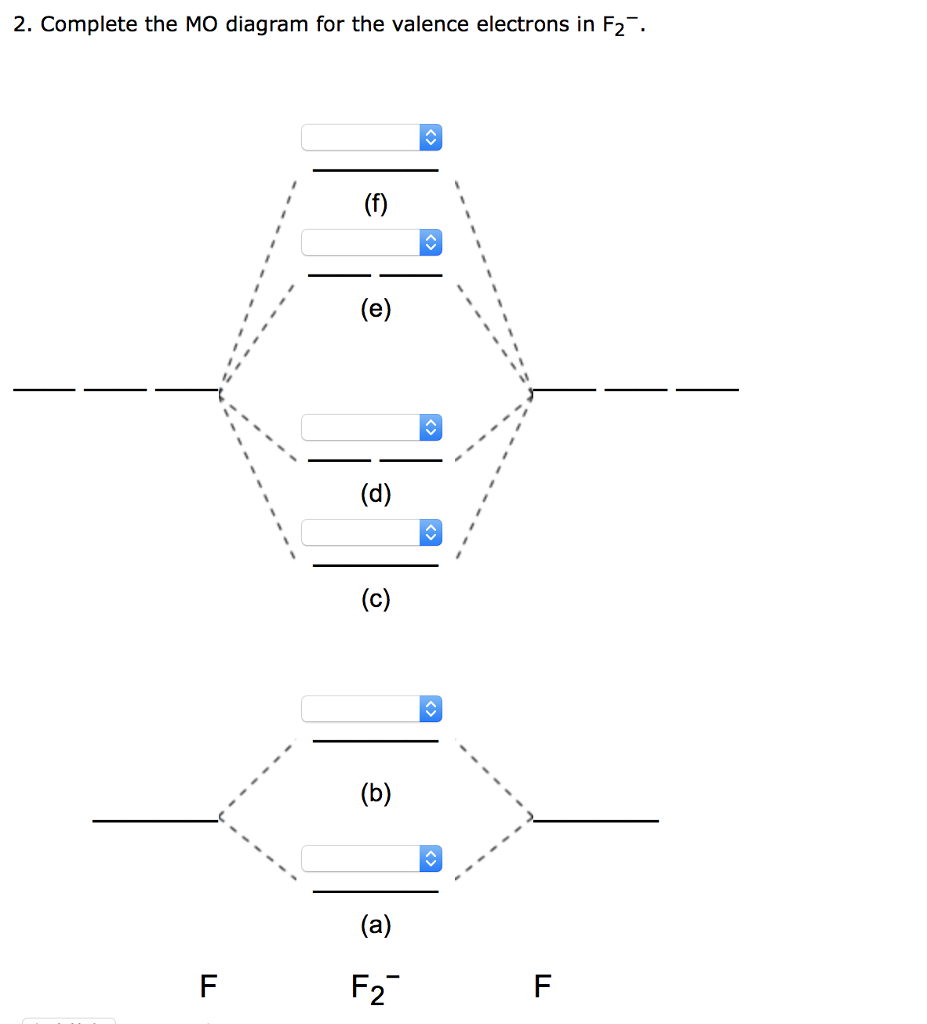
Solved 2. Complete the MO diagram for the valence electrons
MO Diagrams for Diatomic Molecules Chapter 5 Friday, October 9, 2015 Homonuclear Diatomic Molecules What happens when we move to more complicated systems? Consider O2. The Lewis dot structure famously predicts the wrong electronic structure for O2 We can use LCAO-MO theory to get a better picture:
Chemistry Molecular orbital diagrams
Step 1. Start by calculating the number of valence electrons in each atom of F2 and see how many more electrons each fluorine atom needs to form an octet. The atomic number of fluorine is 9; therefore, it possesses 9 electrons in its neutral atomic form. There are 2 electrons in its K shell and 7 electrons in its L shell.

MO diagram for F2+ and F2 Brainly.in
The MO diagram of the valence molecular orbitals can be constructed by combining the valence 2s and valence 2p orbitals from each F atom. The bond order is 1 and the molecule is diamagnetic. This page titled 5.2.1: Molecular Orbitals is shared under a CC BY-SA 4.0 license and was authored, remixed, and/or curated by Kathryn Haas .

Draw molecular orbital of f2 molecule Chemistry Chemical Bonding
Step 1 - Determine the number of valence electrons in total. Since there are now two atoms in the molecule, the total number of valence electrons is double that of the atomic species. Step 2 - Determine the number of electrons in each s and p orbital. Remember that there is one s-orbital and three p-orbitals in the n=2 energy level.